Modified WHO classification of benign bone tumours
- Bone forming tumours
- Osteoma
- Osteoid osteoma
- Osteoblastoma
- Cartilage forming tumours
- Chondroma
- Osteochondroma
- Chondroblastoma
- Chondromyxoid fibroma
- Giant cell tumours
- Marrow tumours (none)
- Vascular tumours
- Haemangioma
- Lymphangioma
- Glomus tumour
- Other connective tissue tumours
- Other tumours (nervous)
- Neurilemmoma
- Neurofibroma
- Unclassified tumours (none)
- Tumour like lesions
- Solitary bone cyst
- Aneurysmal bone cyst
- Metaphyseal fibrous defect
- Eosinophilic granuloma
- Fibrous dysplasia
- Osteofibrous dysplasia
- Myositis ossificans
- Brown tumour of hyperparathyroidism
- Intraosseous epidermoid cyst
- Giant-cell (reparative) granuloma
Enneking classification of benign bone tumours
This system is based on the biological behaviour of benign tumors as suggested by their radiographic findings:
Stage 1
- Latent benign bone tumour
- This does not progress, or heal spontaneously. Does not need operation.
- E.g. non-ossifying fibroma
- Thick, dense reactive bone is present on both xrays and CT.
Stage 2
- Active benign bone tumours. The tumour is actively growing and may cause symptoms and signs.
- This can expand and even deform the bone but is fully confined by the bone. A stage 2 tumor has a thin rim of bone, but remains intracompartmental. However, sometimes weakening of the cortex leads to pathological fracture.
- E.g. giant cell tumour, ABC.
- Operative curettage and bone grafting are commonly used procedures for such tumours.
Stage 3
- An aggressive benign bone tumour
- This invades and destroys the bone and extends into the soft tissue. Little reactive bone.
- Malignancy may be suspected in such instances.
- E.g. aggressive giant cell tumour
- Curettage and bone grafting are options for treatment.
Staging of Bone Tumours
Staging is the process of classifying a tumour with respect to its grade and its local and distant extent in order to estimate the prognosis for the patient and guide treatment.
With musculoskeletal neoplasms (unlike carcinomas), the GRADE of the tumour is a very important prognostic variable in the staging system. It represents the likelihood of metastasis based on histological measures of cell differentiation and growth.
Essentially for exam:
Watch a benign latent lesion
Stage all other lesions
Strategy for evaluation, staging and treatment of a bone tumour
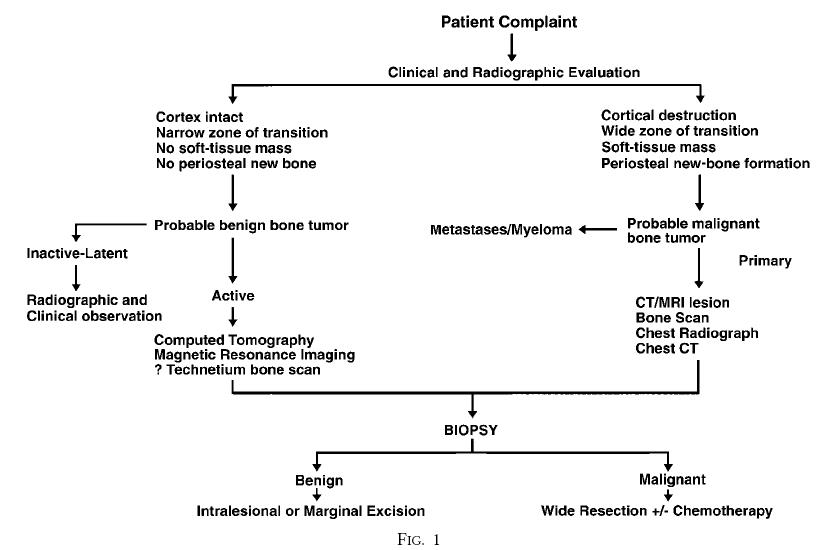
CT scan: good for bone changes, and tumour changes like calcification, ossification.
Must CT lungs to look for mets. The lung is the most common site for mets.
MRI: best for soft-tissue component, skip metastases.
Bone Scanning: looking for mets.
PET etc : Increaseing role for systemic staging, esp. myeloma
Staging:
1. Clinical: neuro, chest, abdo
2. Local Imaging: xray, CT, MRI
3. Systemic Staging: Bone Scan, CXR, CT chest, Blood investigations,+/- PET scan
4. Biopsy
After finding a possible tumour, the patient should be referred for biopsy to the surgeon who ultimately will be responsible for the treatment.
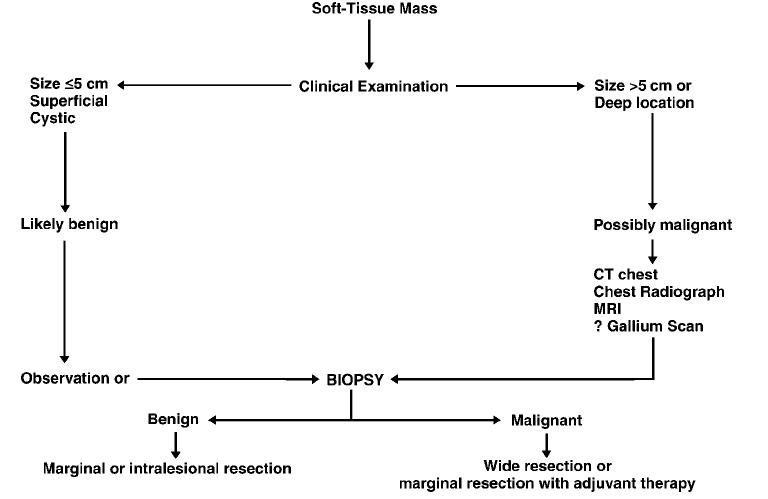
Strategy for evaluation, staging and treatment of a soft-tissue mass
Staging system of Musculoskeletal Tumour Society for Malignant Tumours
Stage I A, B
- All low grade tumours <25% chance of metastasis
Stage II A, B
- All high-grade tumours >25% chance of metastasis
Stage III A, B
- All tumours with metastases
If the neoplasm is intracompartmental it is considered stage A
If it is extracompartmental it is considered stage B
For a BONE tumor to be intracompartmental, it must be within the confines of the cortex or articular cartilage. Any bone tumour extending into the soft tissue is extracompartmental- ie the bone itself is a separate compartment. (Except Parosteal tumours which if still under the fascia/periosteum are still intracompartmental.
This system is applicable to tumours of bone and soft tissue.
It is not applicable to tumours of bone marrow (e.g. lymphoma, leukaemia) which have their own system.
Epidemiology of bone tumours
- In US bone tumours make up 0.2% of all tumours
- Soft tissue tumours make up 2% of all tumours.
- Of primary bone sarcomas:
- Osteosarcoma 35%
- Chondrosarcoma 26%
- Ewing’s tumour 16%
- Chordoma 8%
- MFH and fibrosarcoma 6%
- Angiosarcoma 1%
- Bimodal distribution for bone tumours – second and seventh decade
- Malignant tumours and patient age
1-30 Ewing’s tumour
Osteosarcoma
30-40 Fibrosarcoma and MFH
Malignant giant cell tumour
Reticulum cell sarcoma (primary lymphoma of bone)
Parosteal osteosarcoma
40 plus Mets
Myeloma
Chondrosarcoma - Gradual increase in incidence of soft tissue tumours
- Males > females for bone tumours (except GCT and Parosteal and periosteal osteosarcomas)
- Whites > blacks for bone tumours
Location of tumours within bone
Epiphyseal
- Giant cell tumour
- Chondroblastoma
Metaphyseal
- Osteosarcoma
- Non-ossifying fibroma
- Chondromyxoid fibroma
Metadiaphyseal
- Enchondroma
- Ewing’s tumour
Diaphyseal
- Fibrous dysplasia
- Eosinophilic granuloma
- Ewing’s tumour
Spine
- Aneurysmal bone cyst } Posterior
- Osteoblastoma } elements
Note: If you see a destructive lesion in a vertebral body that DOES penetrate into the disc, then >90% chance it is infection. Tumours do not penetrate the intervertebral disc.
Tibia
- Adamantinoma
- Osteofibrous dysplasia
Radiology of bone tumours
1. Locatio
2. Matrix
3. Zone of Transition
- wide
- narrow
4. Effect on Bone
- Cortex erosion
- gross morphology
5. Periosteal Reaction
6. Extension into soft tissues
Patterns of bone destruction on Xray
- Permeative destruction
- Implies rapid growth
- Lesions insinuate themselves between trabeculae of cancellous bone stimulating osteoclastic activity and fine rarefactions
- Moth eaten
- Implies intermediate aggressiveness
- Coarse destructive pattern
- Geographic bone destruction
- Implies low grade destruction
- Provokes resorption of all osseous tissues in their path
Oncologic margins
Intralesional
The tumour is removed but no attempt is made to obtain normal tissue around it.
Marginal
The tumour is removed completely but the surrounding reactive zone is not completely removed.
Wide
The tumour is removed completely with a surrounding cuff of normal tissue outside the reactive zone.
Radical
The tumour is removed along with the entire compartment in which the tumour lies. E.g., if the distal femur contains the tumour the whole of the femur is removed.
Ablative
Amputation
Types of Biopsy
- Fine needle
- True cut
- Open
- Incisional
- Excisional (rare)
Always culture anything you biopsy, and biopsy anything you culture.
Biopsy remarks
- The biopsy should be performed at the treating institution as rates of complications are five times higher when done at peripheral hospitals, and there are higher rates of amputation and decreased survival. Also, need qualified pathologists to analyse the specimens.
- Antibiotics withheld until cultures are taken
- No esmark bandage if a tourniquet is used.
- The biopsy should traverse only one compartment, and avoid neurovascular structures and joints
- The biopsy tract should be excised at the definitive operation
- The biopsy should be performed at the interface of normal and tumorous tissue. Areas of fracture should be avoided. It is also better to take the specimen from a soft tissue mass rather than bone (minimise chance of fracture)
- Adequacy of biopsy should be checked prior to closure
- Fill bony biopsy site with blue cement
- Haemostasis is mandatory
- Avoid drains: if used, put out through edge of wound
- Try not to use interrupted sutures.
Needle biopsy vs. open biopsy
Advantages of needle biopsy
- Cheaper
- Avoids need for general anaesthetic
- Decreased risk of haematoma
- Can use accurate image guidance (e.g. CT)
- Less morbidity
Disadvantages
- Higher risk of sampling error
- Not enough tissue for special studies such as flow cytometry, gene rearrangement, electron microscopy and immunohisto-chemical staining.
Grading the Biopsy:
This determines whether the tumor is benign of malignant. The samples are graded. A grade of a tumor is a histological measure of its tendency to metastasize. Histological features looked at include:
- Mitotic activity
- Necrosis
- Degree of differentiation
- Nuclear to cytoplasmic ratio
Treatment of Bone Tumours
Limb salvage is more common nowadays with advent of Chemo and Radiotherapy.
Bone sarcoma: Chemotherapy and surgery, +/- Radiotherapy
Soft tissue sarcoma: Surgery, +/- Radiotherapy
Deciding on the appropriate operation is guided less by stage, and more by the anatomical location and local spread of the tumor itself.
For benign bone tumors, an intra-lesional (curettage) procedure is usually performed, however for many grade 2 and 3 benign tumors, the margins of excision within the lesion are extended by power curettage and adjuvants such as phenol, liquid nitrogen and bone cement (thermal effect).
Limb Salvage
For limb salvage to be acceptable, 2 criteria must be met:
1. The survival rates must be similar to amputation.
2. Function at least equivalent to an amputation and prosthesis.
Limb must be: Vascularised, innervated and stable
Both these criteria have been met in studies to date.
Limb salvage is much more common (80%) than amputation (20%), thanks to improvements in chemotherapy, imaging, and reconstructive surgery options.
Chemotherapy is generally given pre-operatively: this is known as neoadjuvant chemotherapy. If it is given post-op, it is known as adjuvant Chemotx. There are many theories as to why neoadjuvant chemo is better, but it has never been proven to be better. Surgery usually occurs 8-12 weeks after Chemotx.
Note for wound healing in the presence of previous radiotherapy (in the long past):
If less than 45G- wound should heal
If >60G- wound will probably not heal.
Adjuvant Therapy: Rads
- External beam radiation can be used for local control of round cell lesions (Ewings, MYE, LYM) and mets
- Adjuvant treatment for soft tissue sarcomas
- May be used with chemo for Ewings
(studies not clear, many favor surgery) - Risk of post-radiation sarcoma 13%
HIGH GRADE LESIONS, poor prognosis
Limb salvage is less attractive than amputation with:
- Pathological fractures, where the adjacent soft tissues are contaminated and there is a higher risk of local recurrence. Patients who have osteosarcoma and local recurrence should go on to immediate amputation.
- Neurovascular involvement is a relative contraindication; vessels can be reconstructed, and in the upper limb tendon transfers or nerve grafts may retain function.
Options for limb salvage include:
- Arthrodesis: Creates stable, painless, durable limb. Should be done esp if extensive muscle resection was required as part of tumor resection. In the knee, the limb is aligned 10 deg of flexion, 0-5 valgus, and 1-2cm shorter in skeletally mature.
- Vascularised bone grafts: Usually fibula. Heal much faster. Undergo hypertrophy and maturation as expected with Wolff’s law.
- Limb lengthening: limited application- bone defects are too large after tumor resection.
- Rotationplasty: Cut out bone from distal femur to above ankle. Rotate distal segment 180 degrees and fuse tibia to distal femur. Ankle now becomes knee joint, and prosthesis fits onto foot. In general, highly functional and long-lasting limb, with relatively few complications. Can have psychological problems adapting to cosmetic appearance.
- Extracorporeal Irradiated autogenous bone
- Modular endoprotheses: may be placed in-situ, combined with allograft or irradiated autograft, or designed as expandable prosthesis. Prostheses have a lower infection rate than allograft. In general though, don’t last that long- 36% at 10 years. The worst performing prosthesis are distal femoral prosthesis in young patients- high rate of aseptic loosening. Proximal tibia does just as bad. Cemented prosthesis in general does better. Infection is the most common reason for an amputation after a limb-sparing operation.
- Osteoarticular allograft: Although associated with more early complications than are endoprosthesis, allograft reconstructions stabilize after 3 to 5 years and therefore do better in long term follow-up studies. With Osteoarticular allografts (articular surface included) OA occurs in 15% at 10 years. In proximal humeral Osteoarticular allografts, there is a high rate of subchondral fractures and collapse of the articular surface.
